Scanning probe microscopy (SPM) allows the visualization
of single protein molecules and their complexes with membranes. In some
cases even visualization of their inner details becomes possible. High
spatial resolution achieved in SPM is not the only advantage of the new
method. Even more important is the possibility to study proteins in various
environments: air with controlled humidity, water and physiologically relevant
buffers, organic solvents. External parameters - temperature, humidity,
pressure, salt concentration - can be varied during measurements as well.
This gives a unique opportunity to study conformational changes in proteins
in situ [1].
Liposomes
Visualization of liposomes, proteoliposomes and
vesicles 10 - 1000 nm in diameter makes it possible to analyze the size
distribution. Processes of liposomes aggregation and fusion can be studied
using SPM [2].
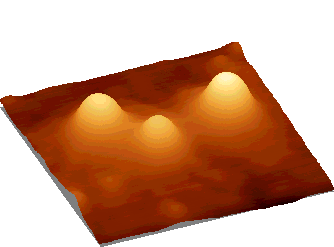
| Fig.
1. Liposomes from phosphatidylcholin on graphite surface. Image
provided by atomic force microscope. The size of individual liposome -
30 nm.
|
 |
Antibodies
Immobilization of antibodies on solid supports
is a primer task for creation of imunnosensors. Scanning probe microscopy
(SPM) proved to be a useful tool for technological control during the preparation
of specifically active films of biomaterials. Films of hepatitis B antibodies
with amphyphylic polyelectrolytes created in the Center of Molecular Diagnostics
and Treatment (Lab. of Dr. I.N. Kurochkin) sensible to HBs antigen were
successfully visualized and quantitatively analyzed [3].
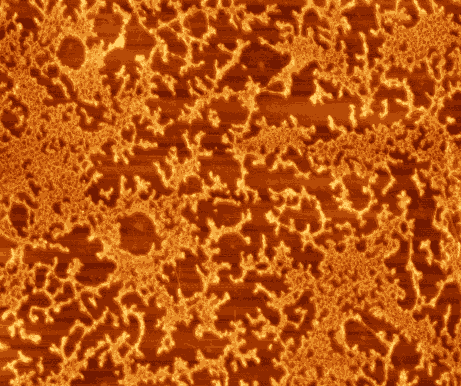
| Fig.
2. Hepatitis B antibodies immobilized on graphite substrate using
polyelectrolyte film. Image size 3.5x3.5 mm2.
|
 |
Self-assembly in protein-membrane systems
Lipids, proteins and other biologically important
substances form liquid crystals and quasicrystalline nanostructures. Visualization
of such formations under various physiological conditions is of great help
in the studies of lipid-protein and protein-protein concurrent interactions
[4]. Self-assembled nanostructures form from membrane proteins, e.g. ATP-syntase
and cytochrome P450 [5].
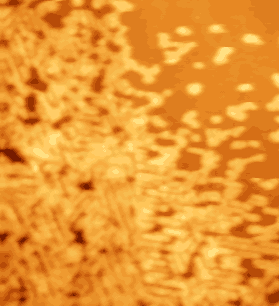 |
Fig. 3. Quasicrystalline formations from ATP-syntase membrane protein complex: nanotubes (image size 2.5x2.5 m m2). |
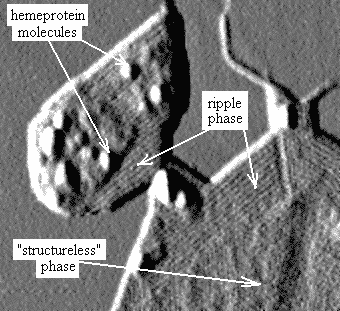 |
Fig. 4. In liquid crystalline so-called "ripple" phase molecules of lipid are arranged in cylinders with parallel orientation (bands on the image). Individual protein molecules (cytochrome P-450 scc) are clearly distinguishable as well. |
Scanning tunneling microscopy
Scanning tunneling microscopy (STM) makes it real
to measure conductivity of biological specimen. It opens perspectives of
registration of charge transfer on single molecule level.
Representative publications:
1. Kiselyova O.I., Yaminsky I.V. "Proteins and
memrane-protein complexes" in Scanning Probe Microscopy of Biopolymers
(Ed. by Yaminsky I.V., Scientific World, Moscow, 1997) p. 41. (in Russian).
2. Uvarov V.Yu., Ivanov Yu.D., Romanov A.N.,
Gallyamov M.O., Kiselyova O.I., Yaminsky I.V. Scanning tunneling microscopy
study of cytochrome P450 2B4 incorporated in proteoliposomes // Biochimie
78 (1996) 780-784.
3. Budashov I.A., Kurochkin I.N., Tsibezov V.V.,
Kalnov S.L., Denisov A.K., Kiselyova O.I., Yaminsky I.V. Structural and
Functional Properties of Langmuir Films from Antibodies based on Amphiphilic
Polyelectrolytes // in press
4. Kiselyova O.I., Yaguzhinsky L.S., Yaminsky
I.V., Yanushin M.F., Bueldt G. Quasicrystalline nanostructures formed on
HOPG surface from ATP-syntase protein complex of thermophylic bacteria
Chlorophlexus aurantiacus // Surface Science in press
5. Kiselyova O.I., Guryev O.L., Krivosheev A.V.,
Usanov S.A., Yaminsky I.V. Atomic force microscopy studies of Langmuir-Blodgett
films of cytochrome P450 scc (CYP11A1): hemeprotein aggregation states
and interaction with lipids // Langmuir in press