(reproduced from: Yuri F. Drygin, Olga A. Bordunova, Marat O. Gallyamov, Igor V. Yaminsky // FEBS Letters 425 (1998) 217-221)
Scanning probe microscopy, which includes scanning tunneling microscopy and atomic-force (or scanning force) microscopy (AFM), was proved to be a reliable, convenient technique for structure investigations of tobacco mosaic virus (TMV) [1,2]. Both methods have unique advantages in the studies of natural [3] or synthetic [4] nucleic acids and specific DNA-protein interactions in air [5,6]. Although there is sufficient information at present about sample preparation for routine DNA investigation by AFM, there are a few reports, to our knowledge, on AFM of the viral high molecular weight RNA molecules [7].
We examine high molecular weight RNA molecules and ribonucleoproteins by the atomic force microscopy. Partial stripping and complete uncoating of TMV virions in mild alkaline conditions, dimethylsulphoxide or urea was carried out to prepare tobacco mosaic virus ribonucleoproteins and RNA for visualization. The AFM images of entire tobacco mosaic virions, partially uncoated TMV particles with protruding RNA molecule from one or both ends and individual TMV RNA molecules were obtained.

Images of TMV particles on pyrographite are presented in Fig. 1 . One can see separate entire particles (about 300 nm length) and shorter and longer particles as well. Broken TMV particles resulted, probably, from the centrifugation process during the sample purification. Longer particles could be the result of TMV virion stacking. The width of TMV particles at the virion half-height is 25-35 nm independent of the mode of investigation. The observed height of tobacco mosaic virions ranged from 17-23 nm in case of the tapping mode to 19-23 nm in case of the contact mode of investigation.
To find RNA molecules deposited onto substrates, partially uncoated TMV particles were used (Fig. 2). Here, the termini of the stripped virus particles served as a starting point for recognizing nascent RNA protruded from the well defined structure.
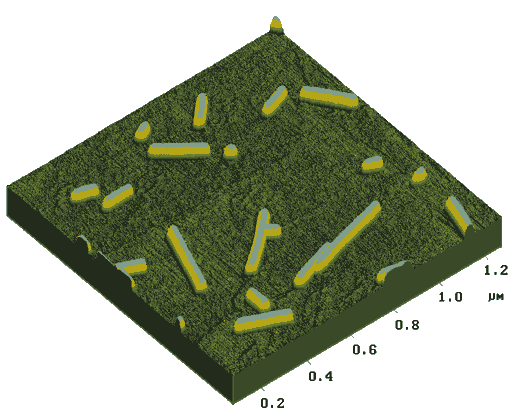
The images of free RNA molecules are presented on Fig. 3.
Measured RNA widths at the molecule half-height ranged from 10-15 nm for the tapping mode to 15-25 nm for the contact mode of investigation. RNA heights are in the range of 0.3-1.5 nm both for the tapping and contact mode investigation.
It is well known that free single stranded RNA is difficult to examine microscopically. Until recently, the best method for RNA visualization was the Kleinshmidt's modified protein monolayer technique (see for example [8,9]) which needs specimen RNA complexed with a basic protein.
Thus, we demonstrated that AFM is an appropriate
technique for investigation of RNA-protein complexes. The study performed
opens new perspectives for the AFM examination of specific interactions
between viral RNA and proteins.
References:
[1] Mantovani, J.G., Allison, D.P., Warmack, R.J., et al. (1990) J. Microsc. 158 (1) 109-116.
[2] Zenhausern, F., Adrian, M., Emch, R., et al. (1992) Ultramicroscopy 42-44, 1168-1172.
File translated from TEX by TTH, version 1.57.
More information see also.
Home pages:
Nucleic acids researches